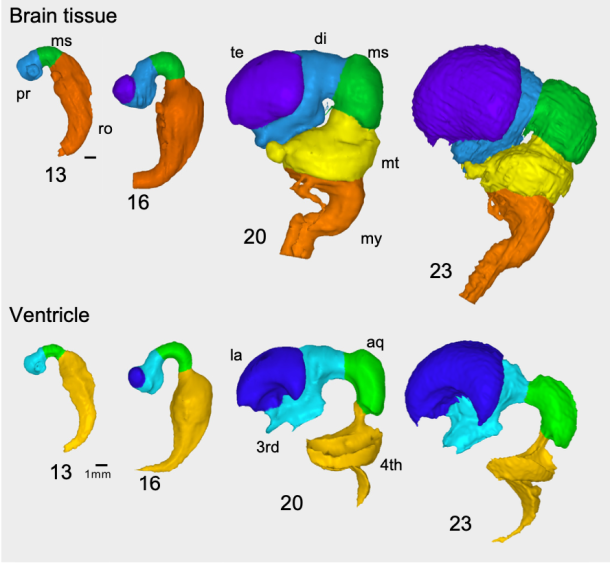
栃木県こども総合科学館に、胚子の脳3Dデータを提供しました。
10月のリニューアルオープンに合わせ、新設されるそうです。
|
||||
|
Warning: Undefined array key "widget_center_top" in /home/kyoto-pathology/pub/hs-kyoto.net/wp-content/themes/atahualpa/index.php on line 11  栃木県こども総合科学館に、胚子の脳3Dデータを提供しました。  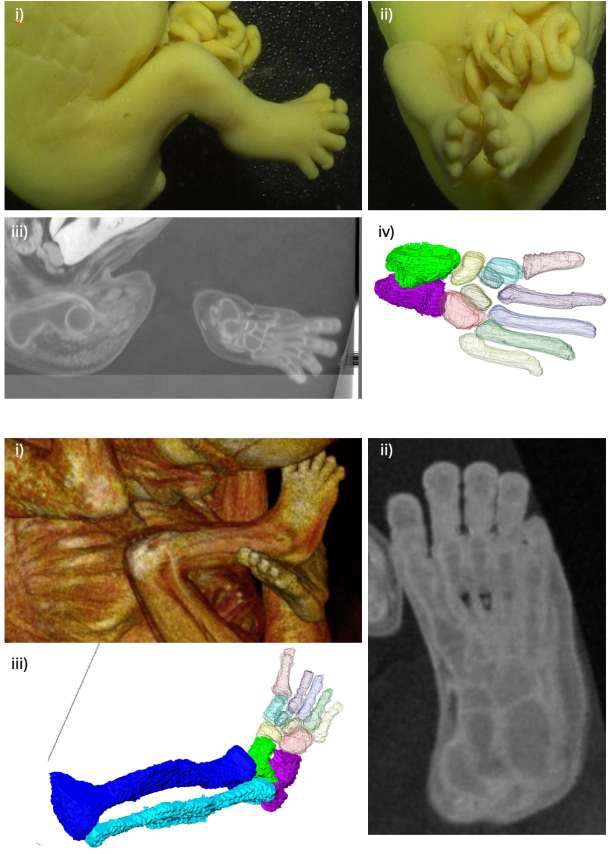 足関節骨格器系の形成についての論文が、Anat Recに受諾されました。胎児期の一時期、足関節が尖足、内反位を示す、生理的内反足を示します。この肢位の正しい理解は、内反足という先天性疾患の理解、診断の向上の面から重要です。
74. Takakuwa T, Matsuda K, Yamato Y, Tamura S, Kimura K, Fujii S, Kanahashi T, Yoneyama A, Imai H, Otani H, Yamada S. Changes in the position of skeletal elements of the ankle and foot during late embryonic and fetal periods. Anat Rec (Hoboken) 2025, in press The morphology of the ankle joint and foot during early development exhibits distinct differences from that observed in adults, with physiological clubfoot being a well-documented phenomenon. To better understand this posture and its transformation, the skeletal elements in this region were three-dimensionally reconstructed using high-resolution phase-contrast X-ray computed tomography and magnetic resonance imaging of specimens (n=23) during the late embryonic and early fetal periods, before joint cavity formation. Sequential changes were analyzed both morphologically and morphometrically from anterior, lateral, posterior, and plantar views. The reduction in plantar flexion of the ankle joint rendered the positional change of the hindfoot substantially more complex, and three-dimensional reconstruction facilitated its comprehension. Continuous supination of the hindfoot, pronation of the forefoot along the foot axis, and reduced plantar flexion of the ankle joint were identified as key postural changes that contributed to the development of temporal physiological clubfoot, initiated as early as the late embryonic period. Twisting between the forefoot and hindfoot and the abduction of the ankle joint, resulting from the obliquity of the tibia–talus joint, were substantial. The offset effect of the two angle changes conceals such changes in most previous studies. Changes in the shape of the tarsus bones, especially the calcaneus and talus, affected the mutual and adjacent bone positions, indicating that the concept of “differential growth” may apply to ankle-joint and foot morphogenesis. Findings of the present study are expected to enhance understanding of the pathogenesis and mechanisms underlying clubfoot and facilitate fetal diagnosis via morphological assessments. 石田さんの修士論文の一部がJ Anatomyに受諾されました CS16からCS23までの合計47個のヒト胚の高解像度の磁気共鳴画像取得し、中腸のヘルニア期に中腸と腸間膜で時間の経過とともに生じる形態学的変化を検討、二次ループと三次ループの初期形成、それに続く追加ループの形成を正確に記載しました。この研究の知見は、ループ形成における遺伝的要因と生体力学的要因の役割を包括的に理解する上で極めて重要です。 70. Ishida N, Ueda Y, Kanahashi T, Matsubayashi J, Imai H, Yamada S, Takakuwa T. Hierarchical loop formation in human midgut during physiological umbilical herniation, J Anatomy 2025, 247, 68-82, DOI: 10.1111/joa.14228 
Abstract The primary loop, a single hairpin-shaped loop, becomes recognizable at the Carnegie stage (CS) 16. This loop projects toward the umbilical cord and subsequently gives rise to four secondary loops in the midgut of human embryos. As development advances, the segments corresponding to each secondary loop further develop into an increasing number of loops, referred to as tertiary loops. The mesenteric leaves and the narrowing parts, which serve as the borders of the secondary loops, remain identifiable throughout the subsequent stages of development. This study aimed to describe the morphological alterations that occur in the midgut and mesentery over time during the herniated phase of the midgut. A total of 47 human embryos between CS16 and CS23 and two fetuses in the physiological umbilical herniated stage were selected for high-resolution magnetic resonance imaging acquisition. Specimens were obtained from the Congenital Anomaly Research Center of Kyoto University. Serial tissue sections obtained from four embryos were subjected to histological observation. The midgut and mesentery were reconstructed in three dimensions, and the resulting morphological changes were observed and analyzed. Formation of the primary loop was observed in all specimens between CS16 and CS18. Secondary loops in the midgut were initially discerned at CS19 in segments 2 and 4 (S2 and S4). The border between S3 and S4 was identified at the apex of the midgut hernia, where traces of the vitelline artery and duct enter the mesentery. At CS21 and later stages of development, the presence of three borders at the exact location delineated by mesenteric narrowing was consistently observed, which resulted in the midgut being divided into four segments in all specimens. The formation of tertiary loops was initially identified at Carnegie stage (CS) 21, occurring in either segment S2 or S3. By CS23, tertiary loops were observed in three segments in most specimens. Notably, the initial formation of tertiary loops in S4 occurred one Carnegie stage later than in S2 or S3. Additionally, the increase in the number of folds and the length per fold in S4 was delayed compared with the number and length of folds observed in both S2 and S3. The number of loops in S1 remained constant (one secondary loop) across all specimens. Upon reaching a critical threshold length, the number of loops exhibited a marked increase, accompanied by rapid elongation in S2, S3, and S4. The number of tertiary loops increased in accordance with the crown-rump length, which exhibited a maximum of 19 tertiary loops in S2 to S4 of the midgut. These findings support the hypothesis that tertiary loops develop biomechanically through the rapid elongation of the midgut and slow growth of the mesentery. This study describes the morphological alterations occurring in the midgut and mesentery over time during the herniated phase of the midgut and provides a comprehensive understanding of the roles of genetic and biomechanical factors in loop formation. 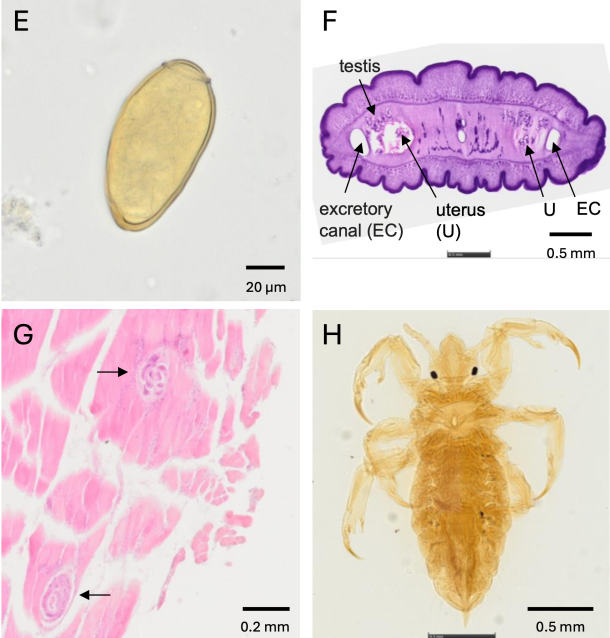 デジタル寄生虫アトラス作成についての論文がSci Reportに受諾されました。希少な寄生虫標本をデータベース化し、教育・研究に役立てる意義のある研究です。 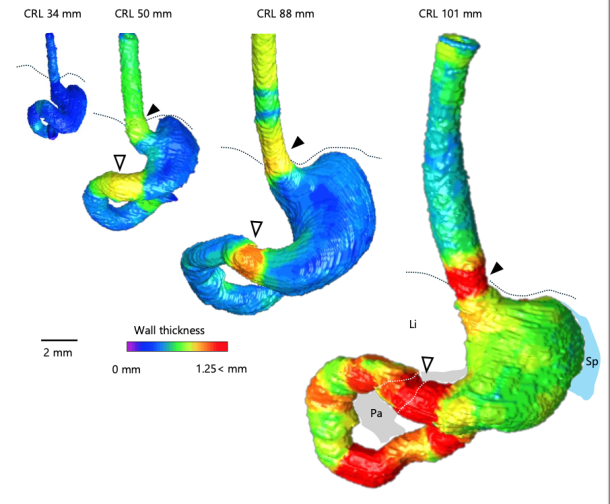 食道、胃、十二指腸の前半部は、尾側前腸から形成されます。その境界部すなわち、食道・胃の境界、幽門部の形成について、MRI画像から管全体、管腔それぞれの太さの評価、Tractographyによる筋層線維形成の評価を行い、特徴を明らかにしました。(金橋先生)
74. KanahashiT, ImaiH, Otani H, YamadaS, Männer J, Takakuwa T. Boundary Formation of the Human Caudal Foregut During the Early Fetal Period: Three-Dimensional Analysis Using T1-Weighted and Diffusion Tensor Images. Cells Tissues Organs, 2025, in press. doi: 10.1159/000546997 Introduction: While caudal foregut development in human fetuses has been outlined in previous research, the formation of its border region remains unclear. This study aimed to visualize the precise timeline of caudal foregut boundary formation. Methods: Three-dimensional images of the foregut from T1-weighted scans of 24 fetuses (crown–rump length [CRL]: 34–103 mm) were analyzed to measure the wall thickness and lumen diameter at nine specific sites. The internal structure in the border region was verified using histological sections and diffusion tensor imaging (DTI) tractography. Results: The lower esophageal and pyloric canal walls in samples with CRL ≥50 mm were relatively thicker. The esophageal wall at the esophageal hiatus, where the lower esophageal sphincter is located, was particularly thick in samples with CRL ≥88 mm. Increased wall thickness at the esophageal hiatus and pyloric canal resulted in a narrower lumen. The pyloric canal lumen narrowed from its distal to proximal sections. The lumen diameter-to-wall thickness ratio at the esophageal hiatus and proximal pyloric was negatively correlated with CRL. The thickened esophageal wall at the esophageal hiatus had a thick submucosa layer, and all layers in the pyloric canal thickened with growth. DTI tractography revealed that the lower esophageal wall mainly comprised longitudinal fibers, whereas the pyloric canal wall consisted solely of circular fibers, with fractional anisotropy increasing with growth. Conclusion: This study provides a comprehensive timeline of normal caudal foregut boundary formation during the early human fetal period, thereby improving the understanding of congenital foregut obstruction pathogenesis. 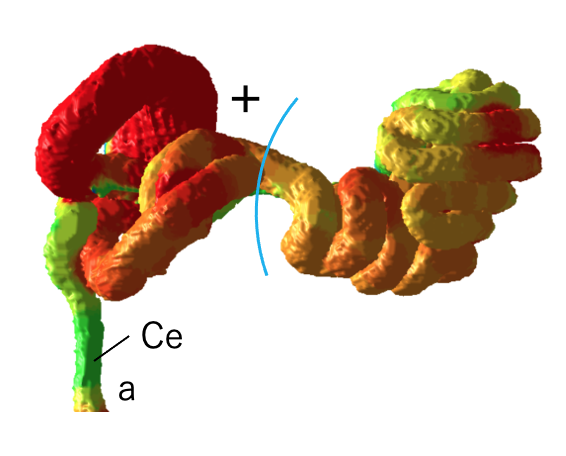 石田さんの修士論文がJ Anatomyに受諾されました。胚子期から胎児期初期の中腸の形成に伴う太さについて検討しました。口側から肛門側へと太さが漸減する様子は美しいです。 Ishida, N, Kanahashi T, Matsubayashi J, Imai, H, Männer J, Yamada S, Takakuwa,T. Change in diameters of the small intestine according to embryonic and early fetal growth. 2025. J Anatomy in press. doi: 10.1111/joa.14285.
Abstract 胚子期ー胎児期初期の上腸管膜動脈の特徴について検討した論文がCells Tissues Organsに受諾されました。掛谷さん、石田さんがおもに解析をした研究です。 中腸ループの臍帯ヘルニア期から還納期の上腸管膜動脈の形態の特徴について、しっかりと検討した論文はこれまでありません。 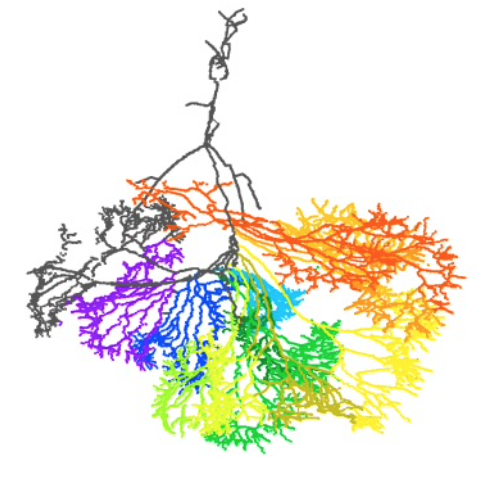
Abstract Introduction: Features of the superior mesenteric artery (SMA) and its intestinal branches during the embryonic and early fetal periods have not been fully described. We aimed to comprehensively elucidate the characteristics of intestinal branch artery formation in the SMA. Takakuwa T, Kakeya M, Ishida N, Kanahashi T, Fujii S, Männer J, Yamada S. Superior mesenteric artery during intestinal loop formation and its positional changes from the extracoelom to the abdominal cavity. Cells Tissues Organs 2025, in press, DOI: 10.1159/000545751  第130回日本解剖学会で発表しました。(2025.3.17-19, 幕張メッセ) ■ 須藤紗帆、松林 潤、金橋 徹、今井 宏彦、大谷 浩、山田重人、高桑徹也;ヒト胚子期・胎児期初期における舌筋発生の検討 (The Development of the Tongue Muscles in the Human Embryonic and Fetal Period) ■ 八田 桃佳、金橋 徹、今井 宏彦、大谷 浩、山田 重人、高桑 徹也;拡散テンソル画像を用いた水晶体線維細胞の配向性の検討 (Analysis of lens fiber cells orientation in human embryonic and early fetal period using diffusion tensor imaging) ■ 石田 七彩、植田 優生、掛谷 真樹、松林 潤、金橋 徹、今井 宏彦、大谷 浩、山田 重人、高桑 徹也;中腸ループ形成を決定する要因:中腸の長さ、直径および位置の影響 (Factors determining midgut loop formation: The impact of midgut length, diameter, and location): 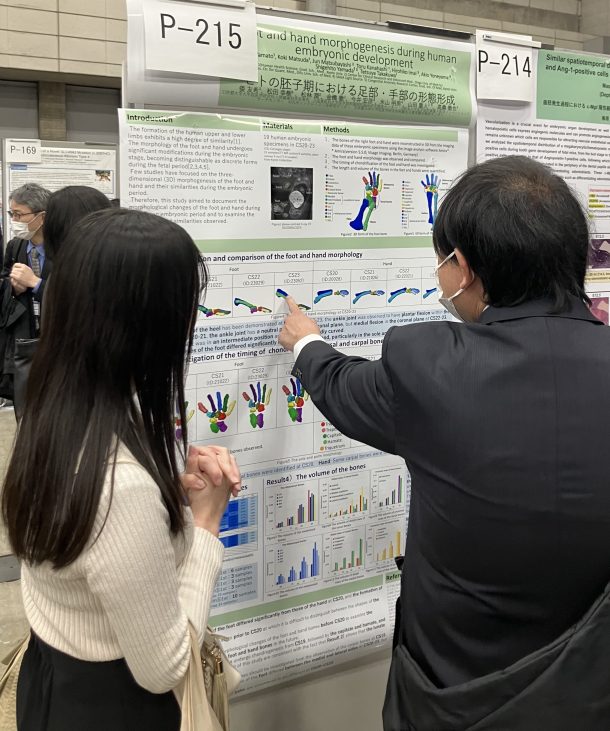 Graduate Student Presentation Award受賞しました!! ■ 熊谷 美優、金橋 徹、今井 宏彦、大谷 浩、多賀 厳太郎、高桑 徹也;高解像度MRIを用いたヒト胎児における大脳基底核原基の形成過程の検討 (Ganglionic Eminences Formation in the Human Fetus) ■ 青江 春菜、金橋 徹、今井 宏彦、大谷 浩、山田 重人、高桑 徹也;ヒト胎児期初期における上顎・下顎・歯胚の三次元解析 (Three-dimensional analysis of maxilla, mandible, and teeth at early human fetal stage) ■ 倭 友希、松田 幸樹、松林 潤、金橋 徹、今井 宏彦、米山 明男、山田 重人、高桑 徹也;ヒトの胚子期における足部・手部の形態形成 (Foot and hand morphogenesis during human embryonic development) ■ 金橋 徹、松林 潤、今井 宏彦、山田 重人、大谷 浩、高桑 徹也;ヒト胎児期初期における骨盤傾斜角の検討 (Analysis of human pelvic tilt angle in the early fetal period) ■ 藤井瀬菜、山田重人、高桑徹也:胚子の体軸がらせん状を描くか否か (Does the embryo’s body axis have a right-handed helical shape? : analysis of 3D reconstructions) Warning: Undefined array key "widget_center_bottom" in /home/kyoto-pathology/pub/hs-kyoto.net/wp-content/themes/atahualpa/index.php on line 51 |
||||