To obtain 3D digital data on early human development, we used some micro-imaging modalities such as magnetic resonance microscopy (MRM), episcopic fluorescence image capture (EFIC), and phase-contrast X-ray computed tomography (PXCT).
2.1 Magnetic resonance microscopy (MRM)
MR microscopy is a very powerful tool for 3D measurements of human embryos, because chemically fixed human embryos contain large quantities of mobile or NMR visible protons, which are major components of the formalin preservation fluid. MR imaging is nondestructive and does not need sectioning of embryonic tissues. Using MRM equipped with superconducting magnets ranging from 1.0T to 7.0T, embryos from the Kyoto Collections were imaged (Haishi et al., 2001, Matsuda et al., 2007, Matsuda et al., 2003, Yamada et al., 2010). The first super-parallel MR microscope was implemented on a 1.5 T clinical MRI scanner operated at University Hospital of Tsukuba University.
Kyoto Human Embryo Visualization Project (BIRD project); this is a collaborative research with Prof. Katsumi Kose of Tsukuba University and has been funded by the “Bioinformatics R & D (BIRD)” Project of the Japan Science and Technology Agency (2005-2010). Kyoto and Tsukuba Universities began a project in 1999 to acquire 3D MR microscopic images of thousands of human embryos using a super-parallel MR microscope operated at 2.35T (Shiota et al., 1991; Matsuda et al., 2003, 2007; Yamada et al., 2006). We were successful in acquiring high-resolution sectional images and in identifying the detailed structures of major organs. A database of developing human embryo images was established for future biomedical research (http://bird.cac.med.kyoto-u.ac.jp). This database will be useful not only in the study of human embryology but also for future gene mapping studies in human development.
Kose and his coworkers (Matsuda et al., 2003) developed a four-channel MR microscope with four unshielded gradient coil probes. The four-channel array probe was developed for a 2.35 T superconducting magnet. Each gradient coil probe consists of a rigid aluminum square frame [18 cm (W) × 20 cm (H) × 28 cm (D)] and slide planes made of thin brass plates (0.3 mm thick) acting as an RF shield. The RF coil units are exchangeable, and the diameter can be optimized for a given sample size. In the project, 18 mm diameter four-turn solenoid coils were used for the 100 MHz signal excitation and detection frequency. The pulse sequence was a T1-weighted 3D gradient echo sequence (TR = 100 ms, TE = 8 ms). Embryo specimens were imaged in test tubes (ID = 13.5 mm) filled with 4% formaldehyde solution. Introduction of a superconducting magnet (2.35 T) to MR microscopy significantly improved the quality of the MR images. The resolution was equivalent to that of low-magnification histological sections. The resolution now approaches 80 µm, and it is possible to identify various embryonic structures, such as the brain (and cerebral cortex), eyes, inner ears, pituitary gland, bronchi, lungs, stomach and intestines, kidneys, gonads, liver and spleen in embryos that are less than 30 mm in length. While introduction of a super-parallel MR microscope enabled imaging of 4-8 specimens simultaneously and significantly reduced the time required (Matsuda et al., 2003).
MRI (7T); MR images were acquired using a 7T MR system (BioSpec 70/20 USR; Bruker Biospin MRI GmbH; Ettlingen, Germany) with a 35-mm-diameter 1H quadrature transmit-receive volume coil (T9988; Bruker Biospin MRI GmbH). The 3D T1-weighted images were acquired using a fast, low-angle shot pulse sequence with the following parameters: repetition time, 30 ms; echo time, 4.037–6.177 ms; flip angle, 40°; field of view, 22.5 × 15.0 × 15.0–42.0 × 28.0 × 28.0 μm3; matrix size, 636 × 424 × 424–768 × 512 × 512; spatial resolution, 35.4–54.7 μm3.
It is feasible to apply MR microscopic imaging to fast and efficient screening of embryos in genetic and teratological studies by identification of internal visceral anomalies quickly, efficiently. MRI is nondestructive and does not need sectioning of specimens, which not only saves time and labor, but also allows precious specimens to remain intact.
2.2 Phase-contrast X-ray computed tomography (PXCT)

Phase-contrast X-ray computed tomography (PXCT) is a relatively newer technique of imaging. In this imaging, the X-rays are used as electric waves which has the information of amplitude and phase. When an X-ray passes through a sample, their amplitude is decreased and phase is shifted. Conventional X-ray imaging (radiography) is based on absorption-contrast (i.e. amplitude imaging) and represents the mass-density distribution of X-ray inside the sample. The sensitivity is not sufficient to perform detailed observations of samples consisting of light elements such as biological soft tissues such as embryos without contrast agent or high X-ray dosages. In contrast, PXCT uses the phase-shift, occurring when X-rays pass through samples (Momose and Fukuda 1995). The sensitivity of the phase shift for light elements such as hydrogen, carbon, nitrogen, and oxygen is about 1000 times larger than that of absorption (Momose and Fukuda, 1995). For phase-shift detection, it is essential to convert the phase shift into the change in X-ray intensity. 2D and 3D observations of various biomedical samples have been performed using synchrotron radiation. The devices using this principle have been developed (Becker and Bonse, 1974, Yoneyama et al., 2004). The 3-D PXCT images of the human embryos were acquired using a radiographic imaging system (BL14-C, 17.8 keV) from Photon Factory, Institute of Materials Structure Science, High Energy Accelerator Research Organization (KEK, Tsukuba, Japan). The data provide a resolution of 18 μm/pixel or better by imaging systems with a two-crystal X-ray interferometer
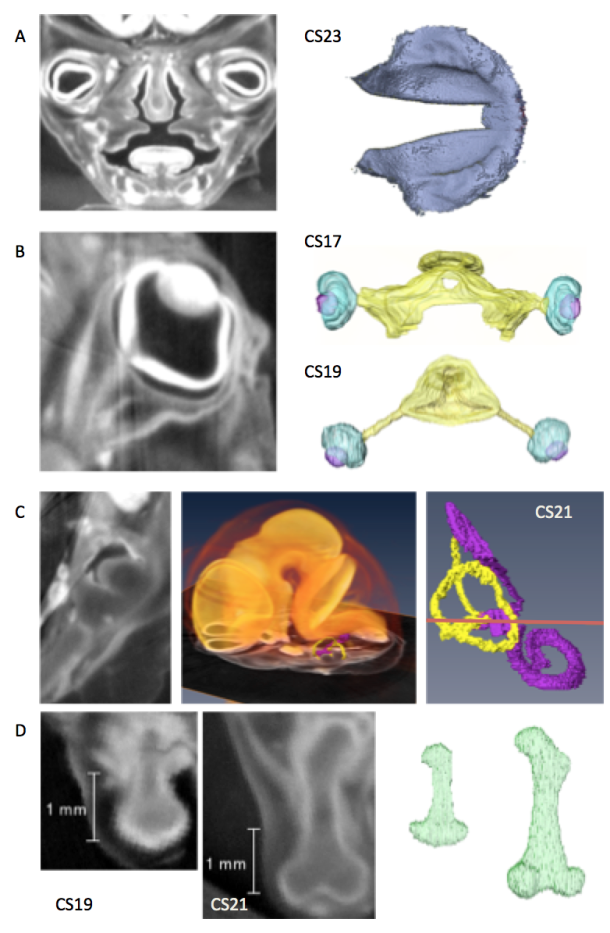
Representative studies using CT images acquired from the Kyoto collection.
CT planes and reconstructed images were provided.
A) Coronal plane of the face and reconstruction of the secondary palate just before fusion (CS23)[1].
B) Sagittal plane of the eyeball and the reconstructions of the eyeball with the optic nerve (CS17 and CS19)[2].
C) Transverse plane of the temporal bone and reconstruction of the inner ear with enlarged brain and inner ear (CS21)[3]
D) Sagittal plane of the thigh and reconstructions of the femur (CS19, CS21)[4]
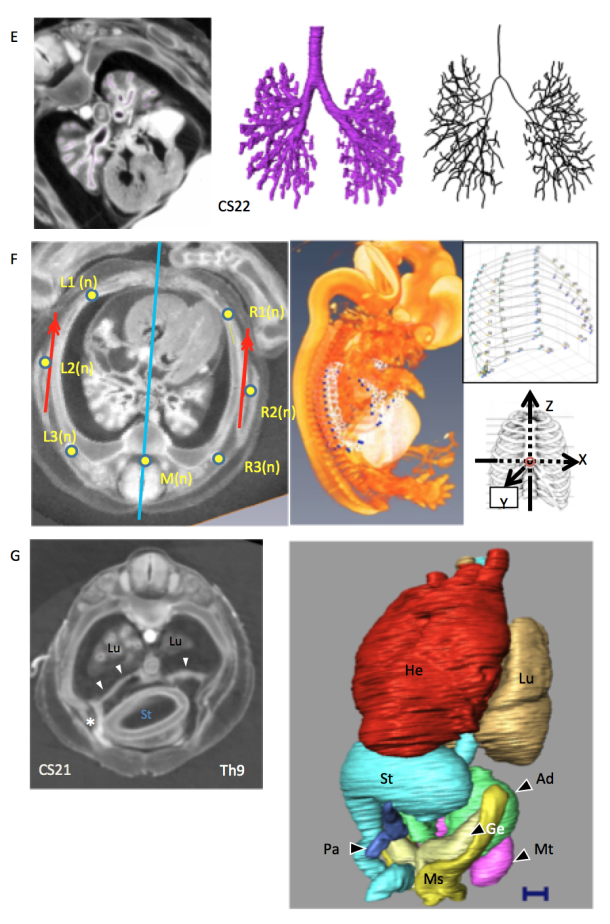
F) transverse plane of the thorax and reconstruction of the whole embryo, thoracic cage, and analysis of the reconstructed image (CS22)[6].
G) Transverse plane of the thorax and abdomen and reconstructions of the thoracic and abdominal organs (CS21)[7]. In this sample, the liver is defective, and the position of organs such as the stomach, pancreas, and heart has been changed.