Abstract. During human development, both external and internal morphological features change dramatically. External features, including those on the body and limbs, provide a good basis for determining the staging of each developing embryo. The application of three-dimensional (3-D) sonography with high-frequency transvaginal transducers has expanded and now fosters 3-D sonoembryology, which provides a basis for assessing normal human development and can also be useful in detecting developmental anomalies. The quantitative data of standard morphology for each Carnegie stage (CS) and early fetal period is required for the evaluation of the body and limbs in clinically obtained data, to allow for better prenatal morphological diagnosis. Analysis of the skeletal system during the human embryonic and early fetal period based will be described, including the rib cage, shoulder girdle, pelvis, and femur. The data obtained may contribute to such evaluations.
Introduction
During human development, both external and internal morphological features change dramatically. External features, including those on the body and limbs, provide a good basis for determining the staging of each developing embryo. For example, flexion and extension of the body combined with the posture of the upper and lower limbs have been integrated into the Carnegie stage (CS), which is universally accepted for determining the staging of human embryos [1]. Although such qualitative external changes are well described in the literature, 3-D quantitative changes in the body and limbs have not been well analyzed. The application of 3-D sonography with high-frequency transvaginal transducers has expanded and now fosters 3-D sonoembryology, which provides a basis for assessing normal human development and can also be useful in detecting developmental anomalies [2,3]. Such technology could contribute to more accurate prenatal diagnoses as well as enable a shift in the diagnostic time window (from the second to first trimester). Under these circumstances, the quantitative data of standard morphology for each CS and early fetal period are required for evaluation of the body and limbs in clinically obtained data, to allow for better prenatal morphological diagnoses. In the present chapter, analysis of the skeletal system during the human embryonic and early fetal period will be described, including the rib cage, shoulder girdle, pelvis, and femur.
Methods
All human embryo and early fetal specimens used are stored at the Congenital Anomaly Research Center of Kyoto University [4,5]. The phase contrast X-ray computed tomography (PXCT) and magnetic resonance imaging (MRI) were used for 3-D data acquisition [6,7]. PXCT and MRI data from selected embryos were analyzed precisely as serial 2-D and reconstructed 3-D images using Amira software (version 5.5; Visage Imaging, Berlin, Germany). The 3-D coordinates were initially assigned by examining the voxel position on 3-D images, which were subjected to principal component (PC) analysis and procrustes analysis.
Results
Rib cage
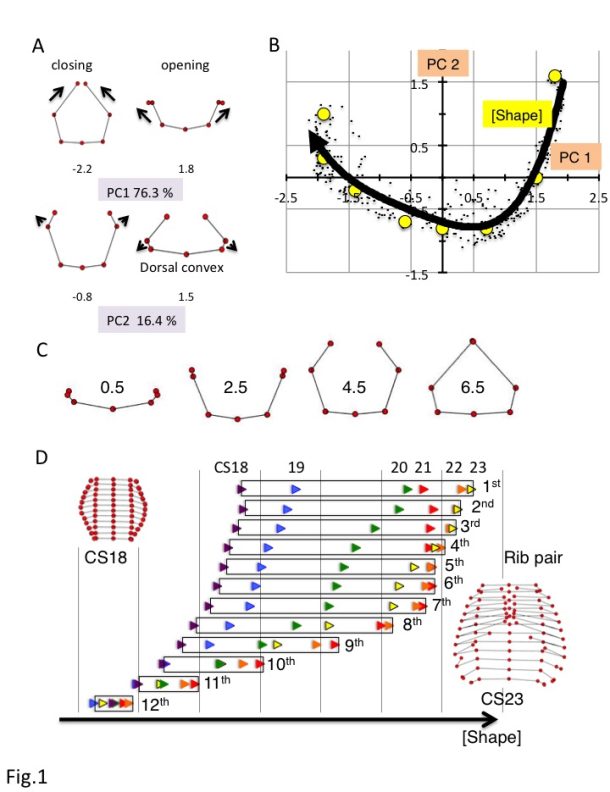
a) Changes in the ribs based on principal component analysis (PC) 1 and PC2. PC scores are indicated below each illustration.
b) Scatter plot of PC1 and PC2 values for all rib pairs (n = 348).
c) Rib morphologies for each F(Shape) value.
d) Changes in F(Shape) according to rib number. CS18, purple; blue, CS19; green, CS20; red, CS21; orange, CS22; yellow, CS23.
The rib cage mainly consists of 12 pairs of ribs and vertebrae as well as the hypaxial muscles on the chest and upper abdominal parts of the body. In the early embryonic period until CS16, all visceral organs, such as the heart, lung, liver, and digestive tract, are covered with soft tissue. However, these organs are not protected by the rib cage because no cartilage or muscle formation is observed until CS16 [1,8]. Such organs are covered by the rib cage until the end of the embryonic period. The rib cage becomes detectable with cartilage formation at CS17, expanding outward from the dorsal side of the chest-abdominal region [9]. The ribs elongate progressively to surround the chest, differentiating into the upper and lower rib cage regions by CS20. The ends of the corresponding ribs in the upper region elongate towards each other, leading to their joining and sternum formation between CS21 and CS23, whereas the lower region of the rib cage remains widely open. The rib cage can be divided anatomically and functionally into the upper and lower thoracic regions. The upper region is related to the pulmonary part of the respiratory system and upper limbs, while the lower thorax is anatomically related to the diaphragmatic part of the respiratory system and also closer to the abdominal cavity and locomotor apparatus [10,11]. We aimed to analyze the morphogenesis of all ribs from the first to the twelfth rib pairs plus vertebrae to compare their differences and features according to the position along the cranial-caudal axis during the human embryonic period [12].
Seven rib cage landmarks, from the first to the twelfth vertebra, and the ribs (for a total of 84 landmarks) were located for each sample. A total of 384 sample data from 32 samples were subjected to process and principal component (PC) analyses, using MATLAB (R2017b, MathWorks, USA) software-assisted algorithms based on orthogonal coordinates of the voxels at each reference point. PC1 and PC2 accounted for 76.3% and 16.4% (sum, 92.7%) of the total variance, respectively, indicating that the change in shape was accounted for by two components (Fig.1A). Changes in PC1 resulted in a circular form surrounding the trunk. A decrease in PC1 showed closing of the rib tips, while an increase in PC1 showed opening of the rib tips. Changes in PC2 showed the movement of the lateral projection and dorsal convexity of the ribs. An increase in PC2 showed posterior movement of the lateral projection at the middle part of the rib, which is related to the dorsal convexity of the ribs, while a decrease in PC2 showed anterior movement of the lateral projection at the middle part of the ribs.
The distribution of scatter plots of the PC1 and PC2 values for each rib showed a fishhook-like shape (Fig.1B). The distribution was fitted to a quartic equation as follows: y = 0.072X4 + 0.1535X3 + 0.1785X2 – 0.2976X – 0.7001; R² = 0.82. PC1 and PC2 plots for each rib moved positions along the fitting curve according to the development of CS18 to CS23. The scatter plots moved in a wide range from the center right (quadrant IV) to the left, reached the left end (quadrant II) of the fitting curve, and moved slightly back in the upper ribs (1-7). However, the scatter plots moved in a narrower range from the center right to the center (quadrant IV) in the lower ribs. Movement was limited to the right (quadrant I) in the 11th and 12th ribs.
PC1 and PC2 values for each rib were plotted close to the fitting curve, for which the shape could be determined using a single parameter. We denote the fitting curve in the principal component subspace in Fig.1B by F(Shape), where Shape is the arc length parameter along the fitting curve and provides a linear scale for shape representation. Thus, the right end of the fitting curve (2.0,1.8) was designated as origin O of F(Shape), while the left end of the curve (-2.3, 1.0) was 6.6. PC1 and PC2 plots by rib x can determine the nearest point X on the fitting curve. The distance XO along the fitting curve was defined as the value of rib x for F(Shape).
F(Shape) = 0.5 showed that the paired ribs were on the dorsal side of the trunk with the opening of the rib tips (Figure 1C). F(Shape) = 2.5 showed that the paired ribs surrounded the dorsal side of the trunk with dorsal convexity of the ribs. The rib tips were separated. F(Shape) = 4.5 showed that the paired ribs had a circular form that surrounded more than half of the trunk, with the lateral projection at the middle part of the ribs. F(Shape) = 6.5 showed that the paired ribs had a circular form surrounding almost all of the trunk with the dorsal convexity of the ribs. The bilateral rib tips were almost closed. The change in F(Shape) at each rib pair is indicated. The development of each rib pair could be indicated as an increase in the F(Shape) scale in almost all conditions (Figure 1D). However, the development of a subset of rib pairs (1st-8th) resulted in a plateau or even decrease in the F(Shape) scale at the end of the embryonic period (between CS22 and CS23).
Our data clearly demonstrated that human embryonic ribs all progress through common morphological forms irrespective of their position on the axis. The data suggested that in a parsimonious model, the common series of rib pairs can be controlled by a small number of factors.
Femur
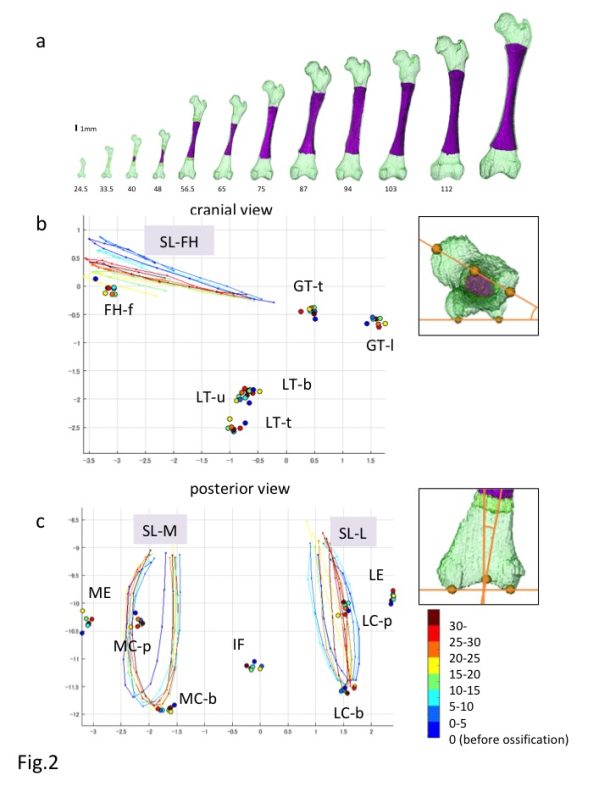
a) Reconstruction of the Femur during development
b,c) Reconstructed Procrustes shape coordinates for the proximal (b) and distal (c) epiphysis of the fetal femur. FH-f: center of the femoral head fovea; GT-1: most lateral point of the greater trochanter; GT-t: top of the greater trochanter; IF: Intercondylar fossa; LC-b: lateral condyle (bottom); LC-p: lateral condyle (posterior); LE: lateral epicondyle; LT-b: bottom end of the lesser trochanter; LT-t: top of the lesser trochanter; LT-u: upper end of the lesser trochanter; MC-b: medial condyle (bottom); MC-p: medial condyle (posterior); ME: medial epicondyle; SL-FH: semi-landmarks from the upper end to the lower end of the femoral head along the plane passing through the midpoint of the femoral head, femoral neck, and greater trochanter; SL-L: semi-landmarks along the roundness of the lateral condyle from the upper end to the opposite side; SL-M; semi-landmarks along the roundness of the medial condyle from the upper end to the opposite side.
The femur is a long bone that develops via endochondral ossification. In particular, the human femur first appears as mesenchymal condensation between CS16 and CS17. Chondrification occurs between CS17 and CS18 and subsequently proceeds to endochondral ossification between CS22 and CS23 [1,8,13]. The cartilage structure influences bone structure formation, as the cartilage structure acts as the blueprint replaced by the bone structure. How the morphological features of the cartilage structure may be replaced by those of the bone structure has not been fully demonstrated, especially precise quantitative information regarding the 3-D formation of the femur.
The morphogenesis and internal differentiation process of the femur were analyzed in 3-D from the fetus (CRL11-185 mm, n = 62) [14]. Procrustes analysis was performed to distinguish the change in shape from the change in size according to growth using defined landmarks (n = 13) and semi-landmarks (n = 3). Centroid sizes at both the proximal and distal epiphyses showed a strong positive correlation with the ossified shaft length (OSL)(R2 = 0.99 and 0.99). The Procrustes shape coordinates for the proximal epiphysis indicated that each landmark on the greater and lesser trochanters and femoral head fovea was located in the same position irrespective of the OSL (Fig. 2A). In comparison, semi-landmarks at femoral head (SL-FH), which lined the femoral head, moved in accordance with the increase in OSL. Procrustes analysis indicated that changes in the femur shape after ossification were limited, and were mainly detected at the time of initial ossification and shortly thereafter. In contrast, femoral neck anteversion and torsion of the femoral head continuously changed during the fetal period. The Procrustes shape coordinates for the distal epiphysis indicated that each landmark was located in the same position irrespective of the OSL (Fig. 2B). semi-landmarks along the lateral and medial condyles (SL-L, SL-M) were located in different positions according to the OSL, although no obvious regularity was noted.
Torsion of the lower leg, including the femur (anteversion), was continuously observed during the fetal period and after birth [15-17]. Torsion of the femur may be affected by repetitive and persistent mechanical forces and the intrauterine position [3]. With respect to mechanical forces, muscle tension and local forces exert a rotary stress on the epiphysis. Remodeling at the metaphysis and epiphysis during the growth of a long bone such as the femur is well known as the mechanism that maintains the shape [8]. Anatomical landmarks remained in the same relative position during subsequent endochondral ossification in the present study, indicating that the remodeling system during femur shaft growth in the longitudinal direction is elaborate.
Shoulder girdle
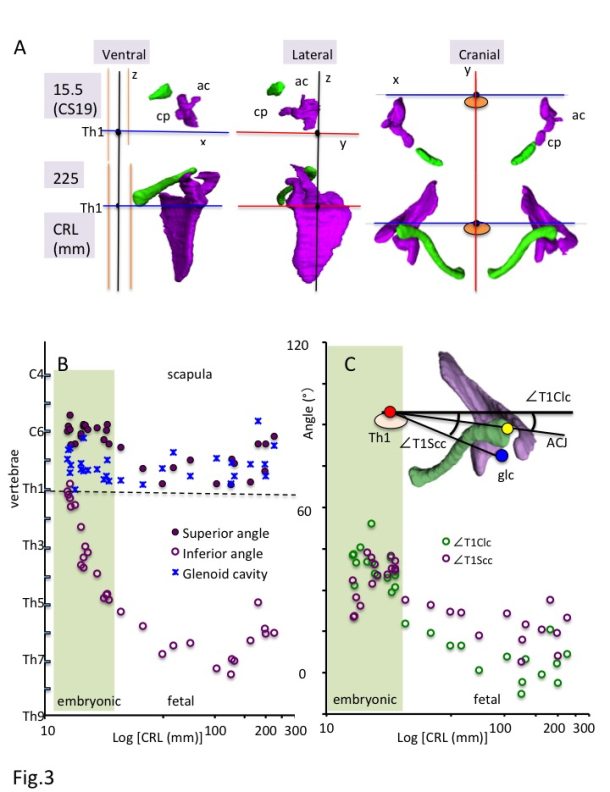
a) Ventral, lateral, and cranial views of the 3-D reconstruction of the scapula (purple) and clavicle (green) in the embryonic (CS19) and fetal (CRL 225 mm) period. Th1: first thoracic vertebra; ac: acromion; cp: Coracoid process.
b) Position of the scapula along z-axis.
Solid purple circles indicate the superior angle of the scapula (spa), open purple circles indicate the inferior angle of the scapula (ifa), and blue stars indicate the center of the glenoid cavity (glc).
c) Relationship between the scapula and clavicle from the cranial view.
Solid yellow circles indicate the angles between the glc-Th1 and ACJ-Th1 segments from the cranial view (∠T1Scc-∠T1Clc).
The 3-D reconstruction and morphometry in our study revealed that all landmarks on the shoulder girdle remained at a similar height except the inferior angle, which means that the scapula enlarges in the caudal direction and reaches the adult position during the fetal period (Fig. 3A-B). The position of the shoulder girdle during the embryonic and fetal periods was unique (Fig. 3A and 3C). In contrast to the constant position of the clavicle, the scapula body was rotated internally and upward at the initiation of the morphogenesis. Thus, the scapula body of both seems almost parallel. The internal rotation of the scapula was changed externally, while the upward rotation remained unchanged. Compared with the adults, the scapula was still rotated internally and upward during the fetal period. The shoulder girdle is located in the ventral part of the body (vertebrae) during the initial morphogenesis, which changes the position to the lateral side of the vertebrae during the late embryonic period and fetal growth. The position during the fetal period may be consistent with that in adults. Such unique positioning of the shoulder girdle may contribute to the stage-specific posture of the upper limb, which is an important external feature for determining the staging, especially between CS18 and CS23 [1]. For example, the axial skeleton (vertebrae) becomes straight and the upper limb extends vertically to the axial skeleton at CS19. The shoulder (humerus head) becomes externally evident, and the joint flexed with the elbow is pronated at CS23. The posture may be explained, in part, by the unique positional change of the scapula during the embryonic period.
Conclusion
Skeletal system analysis may provide a useful standard for morphogenesis and morphometry of the skeletal system, which can serve as the basis to better understand embryonic and early fetal development and aid in differentiating normal and abnormal development.
References
- O’Rahilly R, Muller F. Developmental stages in human embryos: including a revision of Streeter’s horizons and a survey of the Carnegie collection. Washington: Carnegie Institution of Washington; 1987.
- Blaas HG. Detection of structural abnormalities in the first trimester using ultrasound. Best Pract Res Clin Obstet Gynaecol. 2014; 28:341–53. doi: 10.1016/j.bpobgyn.2013.11.004.
- Pooh RK, Shiota K, Kurjak A. Imaging of the human embryo with magnetic resonance imaging microscopy and high-resolution transvaginal 3-dimensional sonography: human embryology in the 21st century. Am J Obste Gynecol. 2011; 204: 77:e1–e16. doi: 10.1016/j.ajog.2010.07.028.
- Nishimura H, Takano K, Tanimura T, Yasuda M. Normal and abnormal development of human embryos: first report of the analysis of 1,213 intact embryos. Teratology. 1968; 1:281–90.
- Yamaguchi Y, Yamada S. The Kyoto collection of human embryos and fetuses: history and recent advancements in modern methods. Cells Tissues Organs. 2018; 205:314–9. doi: 10.1159/000490672.
- Yoneyama A, Yamada S, Takeda T. Fine biomedical imaging using X-ray phase-sensitive technique. In: Gargiulo G, editors. Advanced Biomedical Engineering. Rijeka: InTech; 2011. p 107–128.
- Toyoda S, Shiraki N, Yamada S, Uwabe C, Imai H, Matsuda T, et al. Morphogenesis of the inner ear at different stages of normal human development. Anat Rec. 2015; 298:2081–90. doi: 10.1002/ar.23268.
- O’Rahilly R, Müller F. The skeletal system and limbs. In: O’Rahilly R, Müller F, editors. Human Embryology & Teratology. 3rd ed. New York: Wiley-Liss; 2001. p 357–94.
- Okuno K, Ishizu K, Matsubayashi J, Fujii S, Sakamoto R, Ishikawa A, et al. Rib cage morphogenesis in the human embryo: A detailed three-dimensional analysis. Anat Rec. 2019; 302: 2211-23, doi: 10.1002/ar.24226.
- Bastir M, García-Martínez D, Recheis W, Barash A, Coquerelle M, Rios L, et al. Differential growth and development of the upper and lower human thorax. PLoS One. 2013; 8:e75128. doi: 10.1371/journal.pone.0075128.
- García-Martínez D, Recheis W, Bastir M. Ontogeny of 3D rib curvature and its importance for the understanding of human thorax development. Am J Phys Anthropol. 2016; 159:423–31. doi: 10.1002/ajpa.22893.
- Matsubayashi J, Okuno K, Fuji S, Ishizu K, Yamada S, Yoneyama A, et al. Human embryonic ribs all progress through common morphological forms irrespective of their position on the axis, Dev Dyn. 2019, 248, 1257-63, doi: 10.1002/dvdy.107.
- O’Rahilly R, Gardner E. The timing and sequence of events in the development of the limbs in the human embryo. Anat Embryol. 1975;148: 1-23. doi: 10.1007/BF00315559.
- Suzuki Y, Matsubayashi J, Ji X, Yamada S, Yoneyama A, Imai H, et al. Morphogenesis of the femur at different stages of normal human development, PLoS ONE. 2019;14: e0221569. doi: 10.1371/journal.pone.0221569.
- Felts WJ. The prenatal development of the human femur. Am J Anat. 1954;94: 1-44. doi: 10.1002/aja.1000940102.
- Guidera KJ, Ganey TM, Keneally CR, Ogden JA. The embryology of lower-extremity torsion. Clin Orthop Relat Res. 1994;302: 17-21.
- Gulan G, Matovinović D, Nemeć B, Rubinić D, Ravlić-Gulan J. Femoral neck anteversion: values, development, measurement, common problems. Coll Antropol. 2000;24: 521-527.
- Browne D. Congenital deformities of mechanical origin. Arch Dis Child. 1955;30: 37-41. doi: 10.1136/adc.30.149.37.
- Standring S. Gray’s anatomy. 41st ed. Amsterdam: Elsevier; 2005.
- Schuenke M, Schulte E, Schumacher U, Ross LM, Lamperti ES. THIEME atlas of anatomy